Specialty drugs continue to drive up pharmacy costs, accounting for over 50% of total drug spend for many self-funded health plans, despite being used by a small percentage of patients. But pharmaceutical and administrative solutions are available to help reduce that spend.
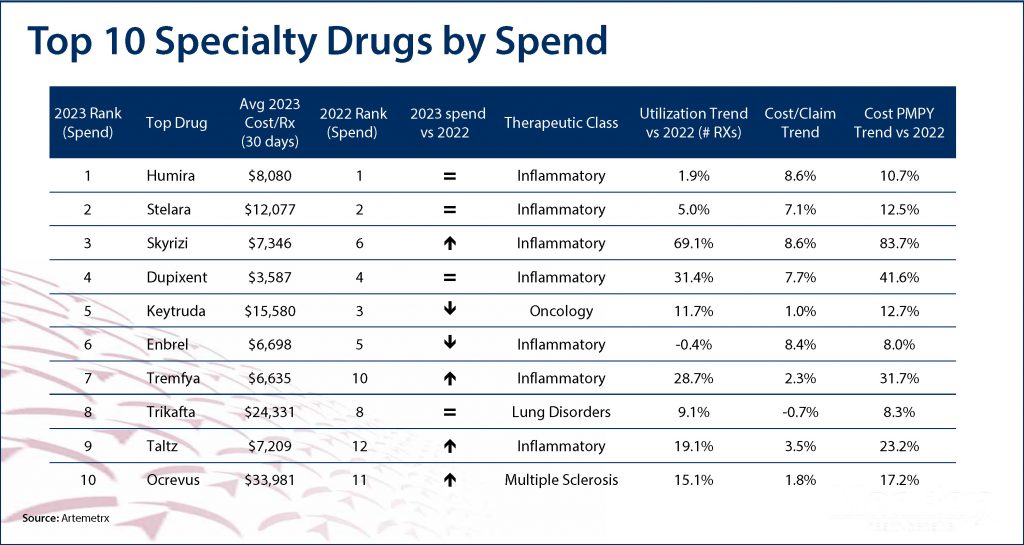
On average, health plans spend $38,000 per year to cover a specialty patient’s drugs, compared to $492 for a non-specialty patient. As the table below shows, in 2023 the average cost of the top 10 specialty drugs was $12,552 for a 30-day supply – primarily for inflammatory disorders like Crohn’s disease and rheumatoid and psoriatic arthritis.
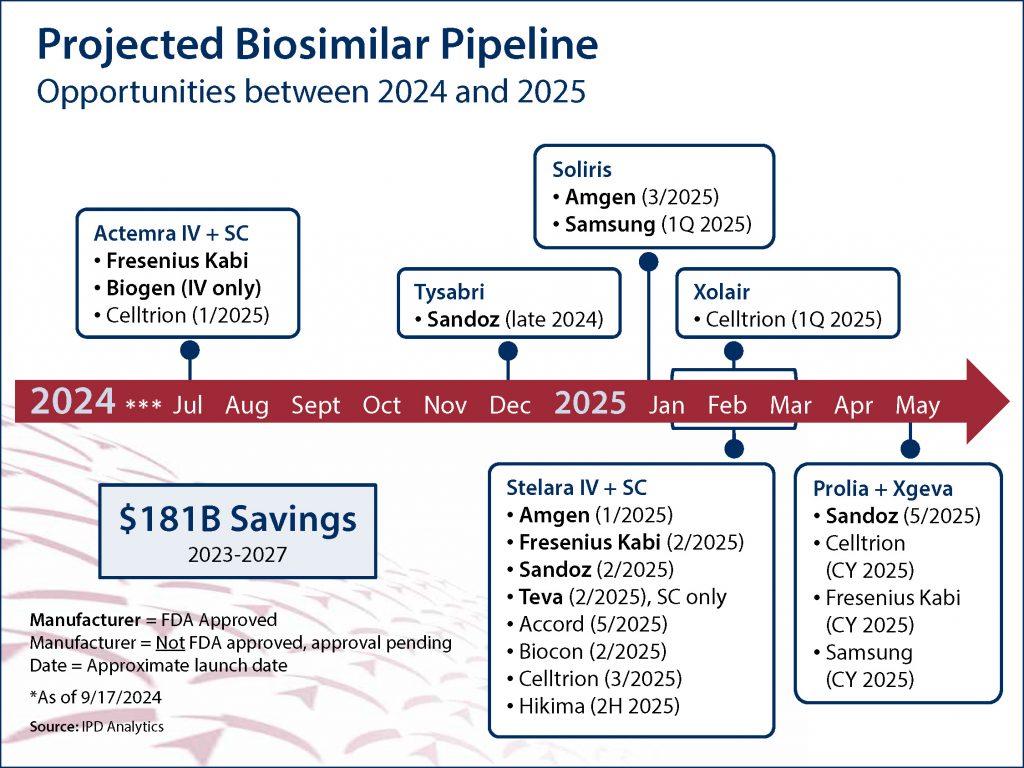
The advent of biosimilars, which are genetically engineered versions of FDA-approved biologic medications, represents a significant opportunity to reduce specialty drug spend. As of October 1, 2024, the FDA has approved 61 biosimilars, and at least 16 more approvals are expected in the first half of 2025 alone (see graphic below).
The economic benefits of biosimilars cannot be overstated. According to recent projections, between 2023 and 2027, their use is expected to generate a staggering $181 billion in U.S. health care savings. Closer to home, MedBen Rx offers biosimilar versions of the inflammatory Humira (which will save clients nearly $3.3 million in 2024) and the insulin Humalog, and we expect to announce additional biosimilars soon.
Additionally, MedBen Rx offers administrative solutions to counter increasingly high drug prices. Through our benefit preservation program, patient advocates work to lower specialty medication costs by utilizing various manufacturer assistance options as well as public and private foundations. We also offer customizable formulary strategies, including over-the-counter medications, discretionary drugs, and specialty pharmacy management.
In short, there are a variety of tools employer plans have to lower their specialty drug spend. Contact your broker or call our VP of Sales & Marketing Brian Fargus at 888-627-8683 to discuss which strategies would work best for you.