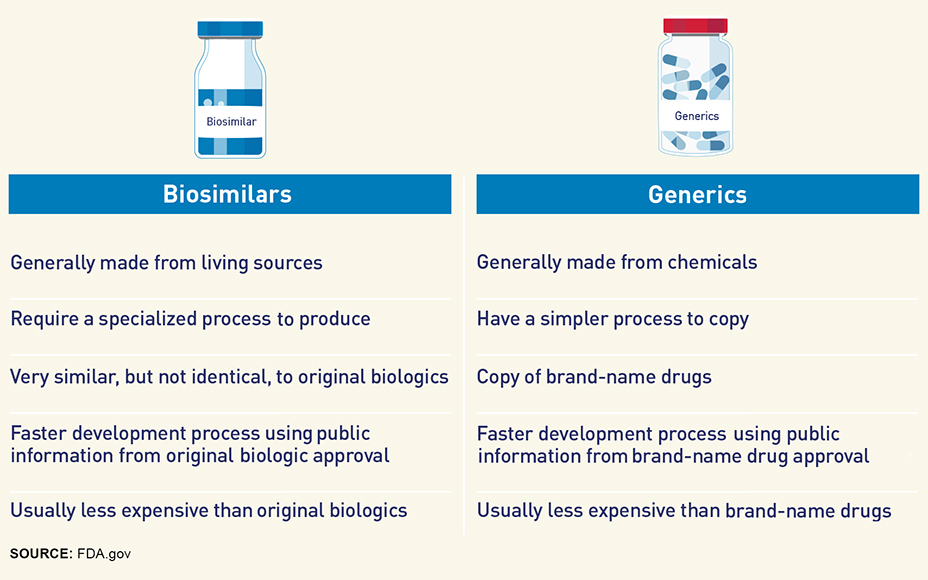
Much like generics are lower-cost equivalents of more expensive drugs, so too are biosimilars. But there’s a notable distinction between the two.
- Generics contain the exact same active ingredients as their brand-name counterparts and are usually synthesized from chemicals. (Inactive ingredients may vary from the brand drug.)
- Biosimilars are less expensive versions of FDA-approved biologic medications – genetically engineered proteins typically manufactured from living cells. Because a biosimilar is made from a living source, it will not be identical to the original biologic, but will still offer the same benefits.
Despite these differences, biosimilars have proven themselves effective alternatives to brand-name drugs. And while biosimilars cost more on average than generics (though less than brand), their increasing popularity should continue to drive their costs down.
The thing biosimilars definitely share with other types of drugs is that they’re big business. Global sales of biosimilars topped $15 million in 2020 – an amount expected to double by 2025. As of December 2022, the FDA has approved 40 biosimilars, with over 100 more in various stages of development.