At January’s Benefits Advisory Conference, MedBen President & CEO Kurt Harden observed that when the Food and Drug Administration (FDA) evaluates a new drug to be approved for sale, it has to be safe and effective, but not necessarily superior to what’s already on the market. “FDA approval for a new drug comes down to two things – it’s better than a placebo and it doesn’t kill you,” he said.
The fact that the FDA has a fairly low bar for drug approval is not widely known, even in the medical community. A Brigham & Women’s study of physician perceptions found that:
- 73% of physicians think (incorrectly) that the FDA only approves new drugs if they are at least as good as existing drugs.
- 70% of physicians think (also incorrectly) that the FDA approves drugs based on clinically meaningful benefits.
So it’s true that “new” doesn’t always mean “better.” It should also be noted, however, that sometimes the most effective drug may happen to be a more expensive one. That’s why comparative effectiveness looks beyond cost… we maximize drug savings by maximizing clinical value.
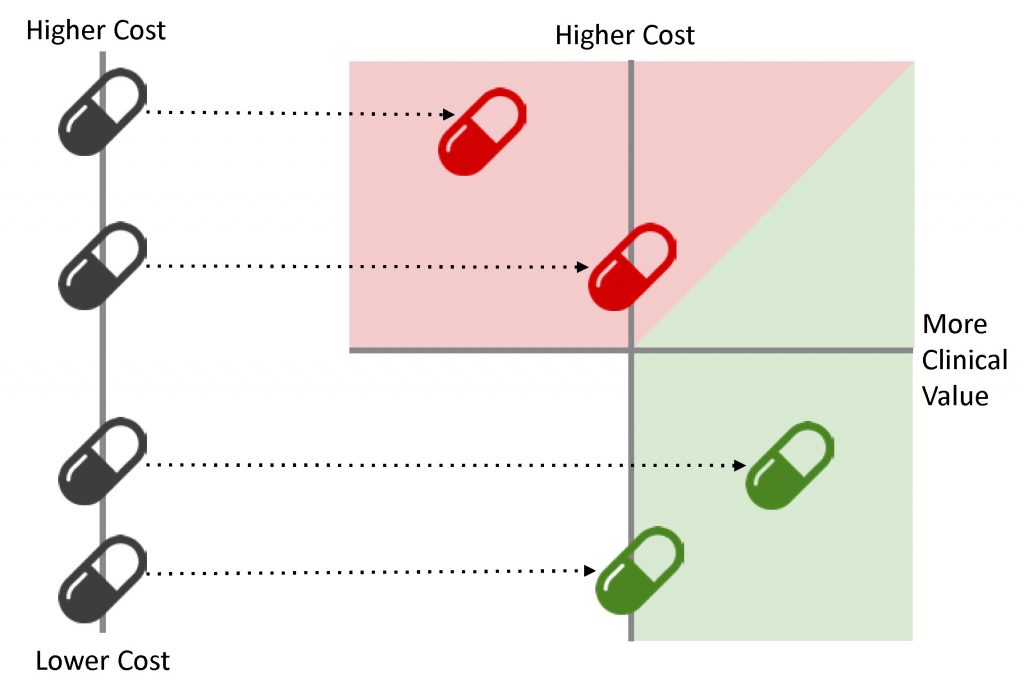
The chart above gives you a general idea of how comparative effectiveness works. Using evidence-based research, we compare drugs created to treat a specific condition based on cost and clinical value factors. Ideally, the drugs that offer the highest value come at a lower cost… but knowing that a pricier drug offers high value is better than paying less for an ineffective medication.
Knowing the cost tells you nothing about a drug’s value, but comparative effectiveness can tell you exactly what you need to know. Call MedBen Rx at 888-627-8683 to learn more.